Gestational Hypertension
| Pregnancy-induced hypertension | |
|---|---|
 | |
| Micrograph showing hypertrophic decidual vasculopathy, the histomorphologic correlate of gestational hypertension. H&E stain. | |
| Specialty | Obstetrics |
Gestational hypertension or pregnancy-induced hypertension (PIH) is the development of new hypertension in a pregnant woman after 20 weeks' gestation without the presence of protein in the urine or other signs of pre-eclampsia.[1] Gestational hypertension is defined as having a blood pressure greater than 140/90 on two occasions at least 6 hours apart.[1]
Signs and symptoms
[edit]No single diagnostic test currently exists to predict the likelihood of developing gestational hypertension. High blood pressure is the major sign in diagnosing gestational hypertension. Some women with gestational hypertension may present asymptomatic, but a number of symptoms are associated with the condition.[2]
Symptoms
- Edema
- Sudden weight gain
- Blurred vision or sensitivity to light
- Nausea and vomiting
- Persistent headaches
- Increased blood pressure
Risk factors
[edit]Maternal causes
- Obesity
- Mothers under 20 or over 40 years old[3]
- Past history of diabetes mellitus, hypertension (particularly gestational hypertension) and renal disease[3]
- Pre-existing hypertension[3]
- Thrombophilias (anti-phospholipid syndrome, protein C/S deficiency, factor V Leiden)
- Having donated a kidney[4]
Pregnancy
- Multiple gestation (twins or triplets, etc.)[3]
- Placental abnormalities:
- Hyperplacentosis: Excessive exposure to chorionic villi
- Placental ischemia
Family history
- Family history of pre-eclampsia
Diagnosis
[edit]Conditions
[edit]There exist several hypertensive states of pregnancy:
- Gestational hypertension
- Gestational hypertension is usually defined as having a blood pressure higher than 140/90 measured on two separate occasions, more than 6 hours apart, without the presence of protein in the urine and diagnosed after 20 weeks of gestation.[5]
- Pre-eclampsia
- Pre-eclampsia is gestational hypertension plus proteinuria (>300 mg of protein in a 24-hour urine sample). Severe pre-eclampsia involves a blood pressure greater than 160/110, with additional medical signs and symptoms. HELLP syndrome is a type of pre-eclampsia. It is a combination of three medical conditions: hemolytic anemia, elevated liver enzymes and low platelet count.[citation needed]
- Eclampsia
- This is when tonic-clonic seizures appear in a pregnant woman with high blood pressure and proteinuria.
Pre-eclampsia and eclampsia are sometimes treated as components of a common syndrome.[6]
Treatment
[edit]There is no specific treatment, but is monitored closely to rapidly identify pre-eclampsia and its life-threatening complications (HELLP syndrome and eclampsia).[citation needed]
Drug treatment options are limited, as many antihypertensives may negatively affect the fetus. ACE inhibitors, angiotensin receptor blockers, and direct renin inhibitors are contraindicated in pregnancy as they are teratogenic. Methyldopa, hydralazine, nifedipine, and labetalol are most commonly used for severe pregnancy hypertension.[7]
The fetus is at increased risk for a variety of life-threatening conditions, including pulmonary hypoplasia (immature lungs). If the dangerous complications appear after the fetus has reached a point of viability, even though still immature, then an early delivery may be warranted to save the lives of both mother and baby. An appropriate plan for labor and delivery includes selection of a hospital with provisions for advanced life support of newborn babies.[citation needed]
Exercise during pregnancy can help prevent hypertension as it can improve not only health for the mother but also the child. Exercise can regulate the energy needs required during pregnancy while also decreasing inflammation. Regular exercise can also lower the increased stress levels associated with pregnancy.[8]
There have been significant findings on how exercising can help reduce the effects of hypertension just after one bout of exercise. Exercising can help reduce hypertension as well as pre-eclampsia and eclampsia. There have been findings in recent studies suggesting that exercising according to recommended guidelines during pregnancy can reduce the risk of developing gestational hypertensive disorder by 30%. [9] The CDC recommends that during pregnancy, the pregnant women should exercise 150 minutes each week specifically focusing on aerobic activity at a moderate intensity. [10]
The acute physiological responses include an increase in cardiac output (CO) of the individual (increased heart rate and stroke volume). This increase in CO can inadvertently maintain the amount of blood going into the muscles, improving functionality of the muscle later. Exercising can also improve systolic and diastolic blood pressure making it easier for blood to pump to the body. Through regular bouts of physical activity, blood pressure can reduce the incidence of hypertension. [11] A recent meta-analysis presented that exercise interventions in pregnant women could reduce both systolic and diastolic blood pressure. The meta-analysis study found that exercise is more likely to reduce the risk of hypertensive disorder of pregnancy in women who are more prone to developing hypertensive disorders of pregnancy. [12] Risk factors that influence the likelihood of developing hypertensive disorders of pregnancy include, a maternal age of 40 or more, pre-pregnancy obesity, excess weight gain during pregnancy and gestational diabetes. [13]
Aerobic exercise has been shown to regulate blood pressure more effectively than resistance training. Compared to having a sedentary lifestyle, being physically active in aerobic exercise for 30-60 minutes 2-7 times a week significantly decreases the risk of gestational hypertension in pregnancy compared to a sedentary individual who is pregnant. [14] It is recommended to see the effects of exercising, that a person should aim for 5-7 days/ week of aerobic exercise. This type of exercise should have an intensity of light to moderate, utilizing ~85% of max heart rate (220-age). Aerobic has shown a decrease in SBP by 5-15mmHg, versus resistance training showing a decrease of only 3-5mmHg. Aerobic exercises such as jogging, rowing, dancing, or hiking can decrease SBP the greatest. The decrease in SBP can regulate the effect of hypertension ensuring the baby will not be harmed. Resistance training takes a toll on the cardiovascular system in untrained individuals, leading to a reluctance in prescription of resistance training for hypertensive reduction purposes. [15]
Evolutionary considerations
[edit]Humans
[edit]Gestational hypertension is one of the most common disorders seen in human pregnancies.[16] Though relatively benign on its own, in roughly half of the cases of gestational hypertension the disorder progresses into pre-eclampsia, a dangerous condition that can prove fatal to expectant mothers.[17] However, gestational hypertension is a condition that is fairly rare to see in other animals. For years, it has been the belief of the scientific community that gestational hypertension and pre-eclampsia were relatively unique to humans, although there has been some recent evidence that other primates can also develop similar conditions, albeit due to different underlying mechanisms.[16] The underlying cause of gestational hypertension in humans is commonly believed to be an improperly implanted placenta. Humans have evolved to have a very invasive placenta to facilitate better oxygen transfer from the mother to the fetus, to support the growth of its large brain.[18]
Origins of the placenta
[edit]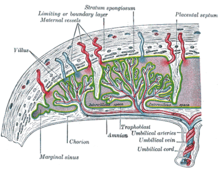
The origins of gestational hypertension may lie with the development of humans' hemochorial placenta. A hemochorial placenta optimizes the amount of oxygen and nutrients that can be absorbed into the fetal blood supply, while at the same time ensuring rapid diffusion of wastes away from the fetus. This hemochorial placenta differs from lower primates' epitheliochorial placentae in the way that it allows the fetal tissues to interact directly with the mother's blood. The hemochorial placenta thereby promotes more rapid diffusion to and from the fetal blood supply.[19]
In animals with epitheliochorial placentae such as horses and pigs, the greatest resistance to maternal blood flow in the vascular system was found within the placenta. However, in animals with hemochorial placental structures such as rodents and primates, the vascular resistance in the placenta was low, leading scientists to the conclusion that the greatest resistance to maternal blood flow is found elsewhere in the maternal vascular system.[20] The high vascular resistance outside of the placenta leads to higher maternal blood pressure throughout the body.[citation needed]
The fetal cells that implant into the uterine wall are known as the trophoblast. The hemochorial placenta bathes the fetal trophoblast in maternal blood by forming lacunae, or lakes, of the mother's blood that surround fetal tissue. The lacunae are filled by the spiral arteries, which means that the mother's blood pressure is the driving force behind the introduction of new blood, which contains both oxygen and food for the fetus, to the system.[21] It is thought that humans need the increased diffusion provided by the hemochorial placenta in order to grow the large brains compared to their body size that distinguish them from other primates.[22]
Incorrect placental implantation
[edit]It is thought that "failings" in normal hemochorial placental structure lead to pre-eclampsia and gestational hypertension.[23] The human placenta implants "earlier, deeper, and more extensively" into the uterine wall, which can potentially lead to many problems that are found in human pregnancies, but not as much in other animals. Miscarriage and pre-eclampsia are both very rare in other species, but are two of the most common pregnancy-related diseases in humans.[24] The genetic roots of gestational hypertension and pre-eclampsia are certain, as women with a family history of the condition are three times more likely to develop it when they are pregnant.[25]
One of the potential causes of gestational hypertension and pre-eclampsia is when the trophoblast does not invade far enough into the uterine lining.[26] When the fetus's trophoblast does not fully extend into the uterine wall, the spiral arteries do not become fully converted into low-resistance channels.[24] It has been found that this incomplete conversion of spiral arteries increases the resistance to uterine blood flow during pregnancy, and that this occurrence was associated with gestational hypertension.[27] One potential cause of this incomplete breach of the spiral arteries that leads to gestational hypertension is a mistaken immune response by the maternal tissue, reaction to the alien fetal tissue.[28] Therefore, it is clear that the complication of gestational hypertension has roots in the early implantation of the fetus in the uterine wall, an implantation technique that is unique to humans.[citation needed]
The highly invasive placenta that is found in humans is thought to be linked to humans' high circulating levels of the hormones CG and hCG. It has been shown that the higher the levels of these hormones, the deeper the trophoblast's invasion into the uterine wall. Instances of gestational hypertension and pre-eclampsia have been shown to occur when the invasion of the uterine wall is not deep enough, because of lower CG and hCG levels in the mother.[29]
Evolutionary tradeoff
[edit]Despite these risks for gestational hypertension, the hemochorial placenta has been favored because of its advantages in the way that it aids in diffusion from mother to fetus later in pregnancy. The bipedal posture that has allowed humans to walk upright has also led to a reduced cardiac output, and it has been suggested that this is what necessitated humans' aggressive early placental structures.[30] Increased maternal blood pressure can attempt to make up for lower cardiac output, ensuring that the fetus's growing brain receives enough oxygen and nutrients.[29] The benefits of being able to walk upright and run on land have outweighed the disadvantages that come from bipedalism, including the placental diseases of pregnancy, such as gestational hypertension. Similarly, the advantages of having a large brain size have outweighed the deleterious effects of having a placenta that does not always convert the spiral arteries effectively, leaving humans vulnerable to contracting gestational hypertension. It is speculated that this was not the case with Neanderthals, and that they died out because their cranial capacity increased too much, and their placentae were not equipped to handle the fetal brain development, leading to widespread pre-eclampsia and maternal and fetal death.[31]
Gestational hypertension in the early stages of pregnancy (trimester 1) has been shown to improve the health of the child both in its first year of life, and its later life.[32] However, when the disease develops later in the pregnancy (subsequent trimesters), or turns into pre-eclampsia, there begin to be detrimental health effects for the fetus, including low birth-weight.[17] It has been proposed that fetal genes designed to increase the mother's blood pressure are so beneficial that they outweigh the potential negative effects that can come from pre-eclampsia.[32] It has also been suggested that gestational hypertension and pre-eclampsia have remained active traits due to the cultural capacity of humans, and the tendency for midwives or helpers to aid in delivering babies.[33]
Relevance of evolutionary history
[edit]It is the goal of evolutionary medicine to find treatments for diseases that are informed by the evolutionary history of a disease. It has been suggested that gestational hypertension is linked to insulin resistance during pregnancy.[34] Both the increase in blood sugar that can lead to gestational diabetes and the increase in blood pressure that can lead to gestational hypertension are mechanisms that mean to optimize the amount of nutrients that can be passed from maternal tissue to fetal tissue. It has been suggested that techniques used to combat insulin insensitivity might also prove beneficial to those with gestational hypertension.[34] Measures to avoid insulin resistance include avoiding obesity before pregnancy, minimizing weight gain during pregnancy, eating foods with low glycemic indices, and exercising.[34]
References
[edit]- ^ a b "40". Williams obstetrics (24th ed.). McGraw-Hill Professional. 2014. ISBN 9780071798938.
- ^ "Gestational Hypertension". www.stanfordchildrens.org. Stanford Medicine Children's Health. Retrieved 2023-10-07.
- ^ a b c d "Gestational Hypertension". Stanford Children's Health. Retrieved 2017-11-30.
- ^ Garg AX, Nevis IF, McArthur E, Sontrop JM, Koval JJ, Lam NN, Hildebrand AM, Reese PP, Storsley L, Gill JS, Segev DL, Habbous S, Bugeja A, Knoll GA, Dipchand C, Monroy-Cuadros M, Lentine KL (January 2015). "Gestational hypertension and preeclampsia in living kidney donors". N. Engl. J. Med. 372 (2): 124–33. doi:10.1056/NEJMoa1408932. PMC 4362716. PMID 25397608.
- ^ Lo, JO; Mission, JF; Caughey, AB (April 2013). "Hypertensive disease of pregnancy and maternal mortality". Current Opinion in Obstetrics and Gynecology. 25 (2): 124–32. doi:10.1097/gco.0b013e32835e0ef5. PMID 23403779. S2CID 246228.
- ^ "preeclampsia/eclampsia" at Dorland's Medical Dictionary
- ^ Brown CM, Garovic VD (March 2014). "Drug Treatment of Hypertension in Pregnancy". Drugs. 74 (3): 283–296. doi:10.1007/s40265-014-0187-7. PMC 4558097. PMID 24554373.
- ^ Witvrouwen, Isabel; Mannaerts, Dominique; Van Berendoncks, An M.; Jacquemyn, Yves; Van Craenenbroeck, Emeline M. (2020-05-08). "The Effect of Exercise Training During Pregnancy to Improve Maternal Vascular Health: Focus on Gestational Hypertensive Disorders". Frontiers in Physiology. 11: 450. doi:10.3389/fphys.2020.00450. ISSN 1664-042X. PMC 7225346. PMID 32457655.
- ^ "PubMed Central (PMC)". PubMed Central (PMC). Retrieved 2024-11-14.
- ^ CDC (2024-10-29). "Pregnant & Postpartum Activity: An Overview". Physical Activity Basics. Retrieved 2024-11-14.
- ^ Ruivo, Jorge A.; Alcântara, Paula (February 2012). "Hipertensão arterial e exercício físico". Revista Portuguesa de Cardiologia (in Portuguese). 31 (2): 151–158. doi:10.1016/j.repc.2011.12.012. PMID 22237005.
- ^ Zhu, Zhu; Xie, Hang; Liu, Shiping; Yang, Ruizhe; Yu, Juan; Yan, Yiping; Wang, Xu; Zhang, Zhihua; Yan, Wu (2022-09-12). "Effects of physical exercise on blood pressure during pregnancy". BMC Public Health. 22 (1): 1733. doi:10.1186/s12889-022-14074-z. ISSN 1471-2458. PMC 9469521. PMID 36096756.
- ^ "PubMed Central (PMC)". PubMed Central (PMC). Retrieved 2024-11-14.
- ^ Magro-Malosso, Elena R.; Saccone, Gabriele; Di Tommaso, Mariarosaria; Roman, Amanda; Berghella, Vincenzo (August 2017). "Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis". Acta Obstetricia et Gynecologica Scandinavica. 96 (8): 921–931. doi:10.1111/aogs.13151. hdl:2158/1079684. ISSN 1600-0412. PMID 28401531.
- ^ "ResearchGATE". SciVee. 2009-01-13. doi:10.4016/9522.01 (inactive 2024-11-12). Retrieved 2024-11-12.
{{cite web}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b Abrams ET, Rutherford JN (2011). "Framing postpartum hemorrhage as a consequence of human placental biology: an evolutionary and comparative perspective". American Anthropologist. 113 (3): 417–30. doi:10.1111/j.1548-1433.2011.01351.x. PMC 3168987. PMID 21909154.
- ^ a b Barton JR, O'brien JM, Bergauer NK, Jacques DL, Sibai BM (April 2001). "Mild gestational hypertension remote from term: progression and outcome". Am. J. Obstet. Gynecol. 184 (5): 979–83. doi:10.1067/mob.2001.112905. PMID 11303208.
- ^ Rosenberg KR, Trevathan WR (December 2007). "An anthropological perspective on the evolutionary context of preeclampsia in humans". J. Reprod. Immunol. 76 (1–2): 91–7. doi:10.1016/j.jri.2007.03.011. PMID 17499857.
- ^ Campbell, Bernard Grant. "Reproduction and the Placenta." Human Evolution: An Introduction to Man's Adaptations. New York: Aldine De Gruyter, 1998. 317-20.
- ^ Moll W, Künzel W (January 1973). "The blood pressure in arteries entering the placentae of guinea pigs, rats, rabbits, and sheep". Pflügers Arch. 338 (2): 125–31. doi:10.1007/bf00592748. PMID 4734441. S2CID 24904753.
- ^ Ahokas RA, McKinney ET (2009). "Development and Physiology of the Placenta and Membranes". The Global Library of Women's Medicine. doi:10.3843/GLOWM.10101. ISSN 1756-2228.
- ^ Martin RD (August 2003). "Human reproduction: a comparative background for medical hypotheses". J. Reprod. Immunol. 59 (2): 111–35. doi:10.1016/s0165-0378(03)00042-1. PMID 12896817.
- ^ Cross JC (2003). "The Genetics of Pre-eclampsia: A Feto-placental or Maternal Problem?". Clinical Genetics. 64 (2): 96–103. doi:10.1034/j.1399-0004.2003.00127.x. PMID 12859402. S2CID 23691148.
- ^ a b Jauniaux E, Poston L, Burton GJ (2006). "Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution". Hum. Reprod. Update. 12 (6): 747–55. doi:10.1093/humupd/dml016. PMC 1876942. PMID 16682385.
- ^ Duckitt K, Harrington D (March 2005). "Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies". BMJ. 330 (7491): 565. doi:10.1136/bmj.38380.674340.E0. PMC 554027. PMID 15743856.
- ^ Norwitz ER (October 2006). "Defective implantation and placentation: laying the blueprint for pregnancy complications". Reprod. Biomed. Online. 13 (4): 591–9. doi:10.1016/s1472-6483(10)60649-9. PMID 17007686.
- ^ Olofsson P, Laurini RN, Marsál K (May 1993). "A high uterine artery pulsatility index reflects a defective development of placental bed spiral arteries in pregnancies complicated by hypertension and fetal growth retardation". Eur. J. Obstet. Gynecol. Reprod. Biol. 49 (3): 161–8. doi:10.1016/0028-2243(93)90265-e. PMID 8405630.
- ^ Robertson WB, Brosens I, Dixon G (1976). "Maternal uterine vascular lesions in the hypertensive complications of pregnancy". Perspect Nephrol Hypertens. 5: 115–27. PMID 1005030.
- ^ a b Cole LA (November 2009). "hCG and hyperglycosylated hCG in the establishment and evolution of hemochorial placentation". J. Reprod. Immunol. 82 (2): 112–18. doi:10.1016/j.jri.2009.04.007. PMID 19560212.
- ^ Rockwell LC, Vargas E, Moore LG (2003). "Human physiological adaptation to pregnancy: inter- and intraspecific perspectives". Am. J. Hum. Biol. 15 (3): 330–41. doi:10.1002/ajhb.10151. PMID 12704709. S2CID 19806255.
- ^ Chaline J (August 2003). "Increased cranial capacity in hominid evolution and preeclampsia". J. Reprod. Immunol. 59 (2): 137–52. doi:10.1016/s0165-0378(03)00043-3. PMID 12896818.
- ^ a b Hollegaard B, Byars SG, Lykke J, Boomsma JJ (2013). "Parent-offspring conflict and the persistence of pregnancy-induced hypertension in modern humans". PLOS ONE. 8 (2): e56821. Bibcode:2013PLoSO...856821H. doi:10.1371/journal.pone.0056821. PMC 3581540. PMID 23451092.
- ^ Rosenberg Karen R.; Trevathan Wenda R. (2007). "An Anthropological Perspective on the Evolutionary Context of Preeclampsia in Humans". Journal of Reproductive Immunology. 76 (1–2): 91–97. doi:10.1016/j.jri.2007.03.011. PMID 17499857.
- ^ a b c Solomon CG, Seely EW (February 2001). "Brief review: hypertension in pregnancy : a manifestation of the insulin resistance syndrome?". Hypertension. 37 (2): 232–9. doi:10.1161/01.hyp.37.2.232. PMID 11230277.
